Thorium to The Rescue
Although the name “thorium” comes from the Norse god of thunder, thorium isn’t as reactive as its namesake suggests, ranking among the least reactive radioactive elements.[51] It is safe to handle in its raw form so long as it’s not ingested, and by itself isn’t particularly remarkable. This lack of natural reactivity and radioactivity, however, is what makes it an ideal fuel in next-generation reactor designs.
As a LFTR is a type of Molten Salt Reactor (MSR), it powers nuclear fission at normal atmospheric pressure through a wholly liquid core that is self-regulating – a completely different setup from the solid fuel rods and pressurized water cores used in traditional nuclear reactors. As discussed previously, meltdowns are problems with solid fuel reactors because a runaway reaction can’t be controlled, which leads to catastrophic results because the solid fuel melts and creates ever-greater pressure until it eventually causes a steam explosion.[52] But an MSR is designed to operate in “meltdown” conditions naturally. In the case of a LFTR, it’s one of the few circumstances in which thorium is sufficiently reactive – and even then, it’s a slow and steady reaction.
Compare LFTRs and Pressurized Water Reactors to the fable of the tortoise and the hare, and you’re on the right track.
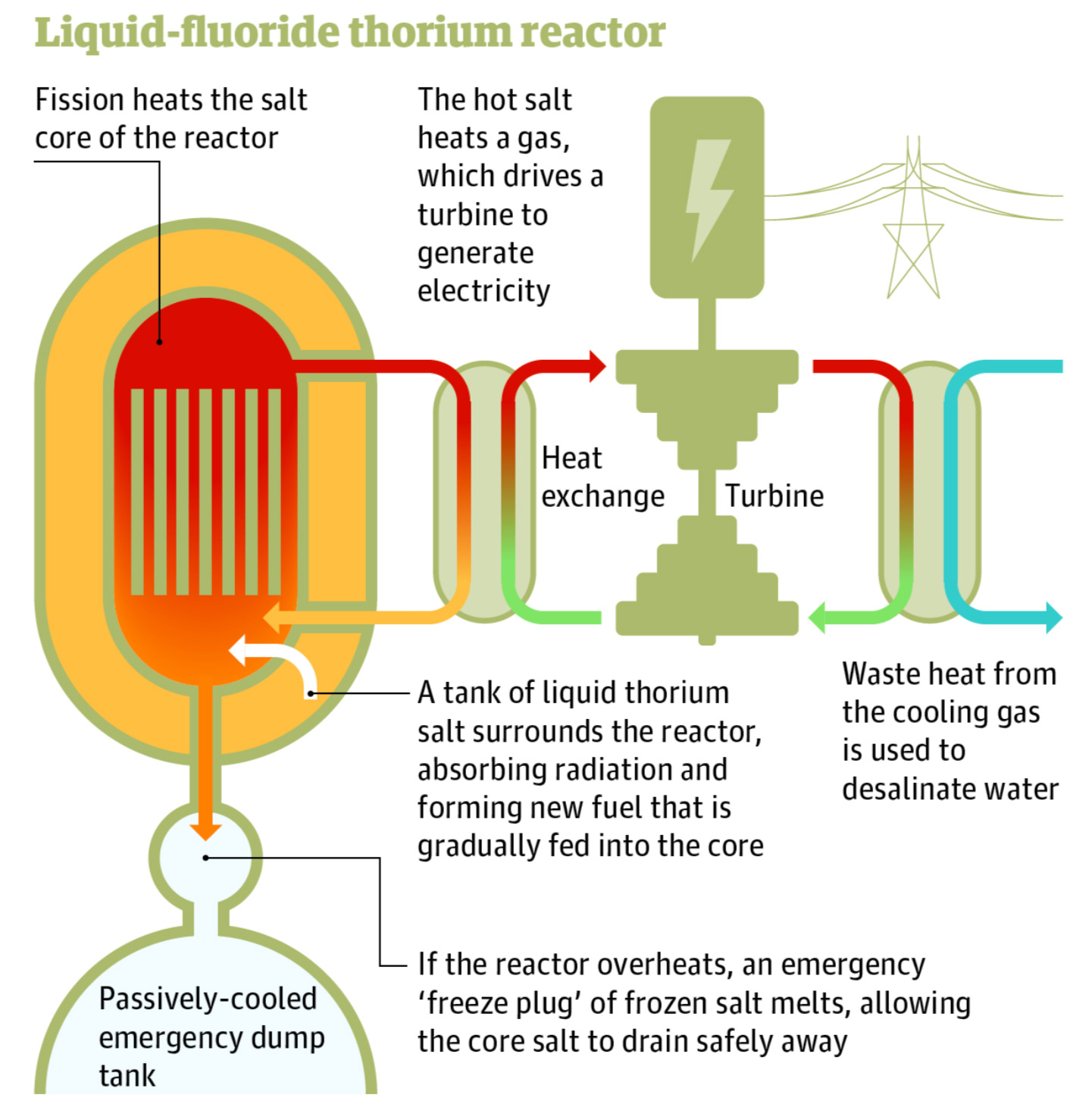
The reaction works like this: thorium-232 and uranium-233 – the kind of uranium that's difficult to use in bombs – are dissolved into molten salts (usually lithium fluoride – but could be any number of alternatives)[54] and fed into the reactor. The molten salts act as a carrier for the thorium fuel and as a catalyst for the reaction, which keeps the fuel supply at high temperature and at the same time helps refuel the reactor over time through breeding.[55]
More technically, a LFTR’s core fissions uranium-233, releasing heat, energy and excess neutrons that combine with the fertile thorium-232 in the liquid molten salt to form more fissile uranium-233 through transmutation. Then, the newly-transmuted uranium-233 is fed back into the reactor core, making for sustained, efficient, and self-regulating fission.[57] This fission reaction, through a series of heat exchangers, then heats an inert gas that is sent through turbines to generate electricity. As this reaction generates lots of heat, there remains plenty of leftover energy that can also be used for on-site water desalination and hydrogen production, which we’ll discuss in the next few chapters.
As LFTRs can reprocess and resupply their own fuel from the waste products of the original fission reaction, in addition to a hefty supply of fertile thorium (known as the “blanket”), LFTRs essentially refuel themselves for long periods of time at high efficiencies. Just how efficient are LFTRs? One ton of thorium-232 in a LFTR gives us the energy equivalent of 250 tons of uranium-235 in a traditional Light Water Reactor, or 4.16 million tons of coal in a coal-power plant.[58]
The breeding process can allow the reactor to continually reprocess and produce its own fuel from thorium for up to 30 years without replacement (although this time would be likely be shorter due to the eventual need to replace certain components of the reactor core).[59] For a fuel supply, though, that’s still some 15 times longer than traditional Light Water Reactors.[60] Plus, LFTRs are 54% efficient (some 20% higher than most coal plants) and use 99% of their fuel.[61] And as that 46% efficiency loss primarily takes the form of heat, we can re-capture that heat to power auxiliary systems that produce resources.
The use of thorium-232 in LFTRs is superior to the use of uranium-235 in a Pressurized Water Reactor in other ways as well, particularly when looking at safety, sustainability, scalability, security and cost.
Safety. Because LFTRs operate at normal atmospheric pressure, far less can go wrong. And in case of emergencies, the fix is simple and effective: gravity. As the reactant is liquid, it can drain into smaller storage tanks with insufficient critical mass to sustain the reaction, thus it freezes.[62] This makes it physically impossible for a LFTR to “melt down” in the traditional sense, even under catastrophic circumstances.[63] If a LFTR was targeted by terrorist attacks and blown up, the liquid reactant would flash-freeze into a solid once exposed to the open air. Additionally, once its fuel cycle has completed, LFTRs produce substantially less radioactive waste than Pressurized Water Reactors.[64] The radioactive waste that remains is also short-lived – staying dangerous only for decades as opposed to millennia.[65]
Thorium is plentiful and sustainable for long-term use. About as common as lead, the global supply of thorium is three to four times greater than all forms of uranium – and only 0.7% of all uranium is fissile.[66] Thorium is also a common byproduct of rare earth metal mining, presenting straightforward opportunities in the short-term for easier acquisition.[67] There is enough thorium in the United States alone to power the country for the next 10,000 years.[68]
LFTRs have a greatly reduced environmental footprint. By nature of their operation, most radioactive waste inside LFTR cores is consumed by the reactor.[69] This enables LFTRs to consume other types of waste as well, including weapons-grade fissile material and even the nuclear waste generated by traditional nuclear reactors.[70] In turn, LFTRs can then act as nuclear garbage disposals that also generate electricity for decades.[71] The physical amount of waste remaining once the reaction consumes all fuel is less than 1/1000th of the waste produced by Light Water Reactors.[72] Additionally, LFTR waste decays quickly, with the most toxic radioactive isotopes having a half-life of only 30.17 years.[73] This means that a supply of radioactive waste from a LFTR would become less radioactive than natural uranium within a period of 300 years or less.[74] More toxic radioactive waste from Light Water Reactors can last for thousands of years.[75]