The Unholy Alliance: Electricity and Bombs
Most nuclear reactors, including those within the United States, are fueled by uranium-235, an isotope representing less than 0.7% of all naturally existing uranium on Earth.[33] Uranium-235 is a fissile fuel, meaning that the possibility exists for its atomic nucleus to split into isotopes of other elements if hit by a fast-moving neutron, releasing levels of energy that are millions of times greater than any known chemical fuel source. For reference: burning a single molecule of methane releases 9.6 eV (electron volts) of energy.[34] Fissioning a single uranium-235 atom releases 200 MeV (million electron volts) of energy.[35] That’s a huge difference.
For nuclear fission to work for power generation, it involves a concept known as “criticality”: a threshold, or “critical mass,” where there is enough fissionable material present for the reaction to sustain itself. As it exists in nature, elemental uranium is not capable of doing this. Yet the isotopes uranium-233 and uranium-235 are. If these isotopes are extracted and placed in a controlled environment (or if enriched into a fuel supply) the fission reaction becomes sustainable over long periods of time. Within Pressurized Water Reactors, this reaction efficiently produces heat, which boils water into steam that turns a turbine and generates electricity. In concept, traditional nuclear reactors are just a very efficient and sophisticated implementation of steam power.
But for several reasons, this past approach is less than ideal. For starters, the uranium-235 fuel cycle is more reactive than thorium and harder to control once it reaches criticality. Further, its sustainability as a fuel source is limited, and spent fuel rods must be replaced (along with the reactor core) every 18-24 months[36] – requiring reactor shutdown. As spent fuel is highly radioactive and contaminates anything it comes in contact with (including disposal equipment), this process creates an effectively endless supply of radioactive waste. If that wasn’t enough, Pressurized Water Reactors can present extremely dangerous conditions if any part of the reaction became unstable or uncontrollable.
Pressurized Water Reactors were invented in the 1950’s and their designs have remained conceptually consistent since then. Most work just fine. Yet should key systems fail, the uranium-235 fuel supply (which is normally placed in extractible rods)[37] could remain stuck inside the reactor. Should this happen, the reaction would continue unrestrained, generating enough heat to melt the fuel supply and cause it to pool at the bottom of the reactor’s pressurized water core. In this circumstance, the reaction would accelerate exponentially to amplify heat production and water pressure until it eventually caused a steam explosion – resulting in the spread of highly radioactive material over a region. That event is called a “meltdown” and is effectively what happened in Chernobyl.[38] Needless to say, such events are catastrophic beyond hyperbole.
Because of the risk of meltdowns, however unlikely, Pressurized Water Reactors must be built with extensive safety features: containment domes of steel-reinforced concrete that are several feet thick, massive cooling and pressurization apparatuses, and redundant mechanisms that engage in case any systems were to fail. Pressurized Water Reactors also must be built in sparsely populated areas with large buffer zones in case the surrounding region needed to be evacuated in an emergency. The expenses and security concerns of this reality prompt an important question, though: why in the heck are we using uranium-235 within Pressurized Water Reactors, even though better alternatives exist?
Simply stated? Because at the end of the day, the uranium-235 fuel cycle is mankind’s best-known pathway to building nuclear weapons.
During World War II, scientists working for the U.S. government (and Third Reich)[39] discovered that certain fissile isotopes had a unique property: if enriched highly enough, they could reach a super-critical state. And if, in this super-critical state, they were rapidly bombarded with neutrons, it could create a nuclear detonation – resulting in the most powerful man-made force in existence.
As it was two of those detonations that ended World War II, the significance of atomic weaponry could not be downplayed, especially once the Cold War unfolded. Thus, as civilian nuclear power developed as an energy source, so did the development of nuclear arms and their delivery mechanisms. These two sectors converged to ensure our continued use of uranium-235 as fuel.[40] But not just because its highly enriched forms are far more powerful than conventional explosives in weapons of war. The other, more essential reason is that the uranium-235 fuel cycle can be leveraged to artificially create plutonium-239 – a far more potent weapons fuel that does not naturally exist on Earth in any significant quantity.[41]
And you need plutonium-239 to build hydrogen bombs.
Nuclear bombs come in varied shapes and sizes. With the right materials, building a basic nuclear weapon is, in theory, relatively straightforward. The general idea is to:
- Find a way to rapidly combine highly enriched fissile material together into a critical mass (explosives usually do the trick),
- Introduce a high-intensity neutron source to spark a fast-fissile chain-reaction, and
- Tada! – you’ve got yourself a nuclear bomb. (Please don’t actually try this at home).
The following image shows a nuclear weapon similar to the description above – a gun-type assembly weapon, which is the bomb the United States dropped on Hiroshima. It’s fairly simple, conceptually speaking.
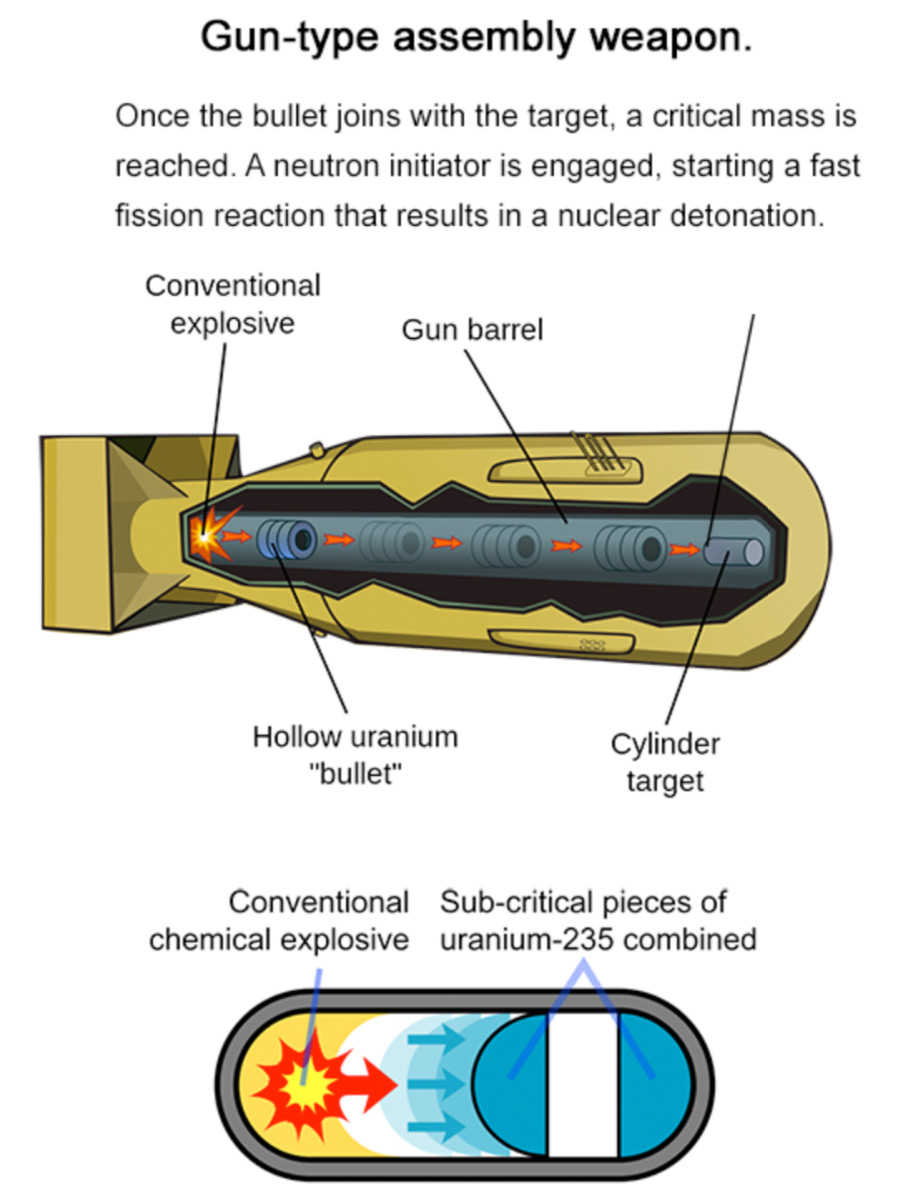
However, building a powerful bomb that’s still compact enough to function as a missile’s warhead requires an implosion-type assembly. This method delivers a critical mass through implosion – compressing a larger sphere of fissile fuel into a smaller sphere by means of explosives – a highly sophisticated and difficult process. Implosion-type devices can not only be significantly smaller than gun-type devices, they are also far more efficient and thus far more destructive.
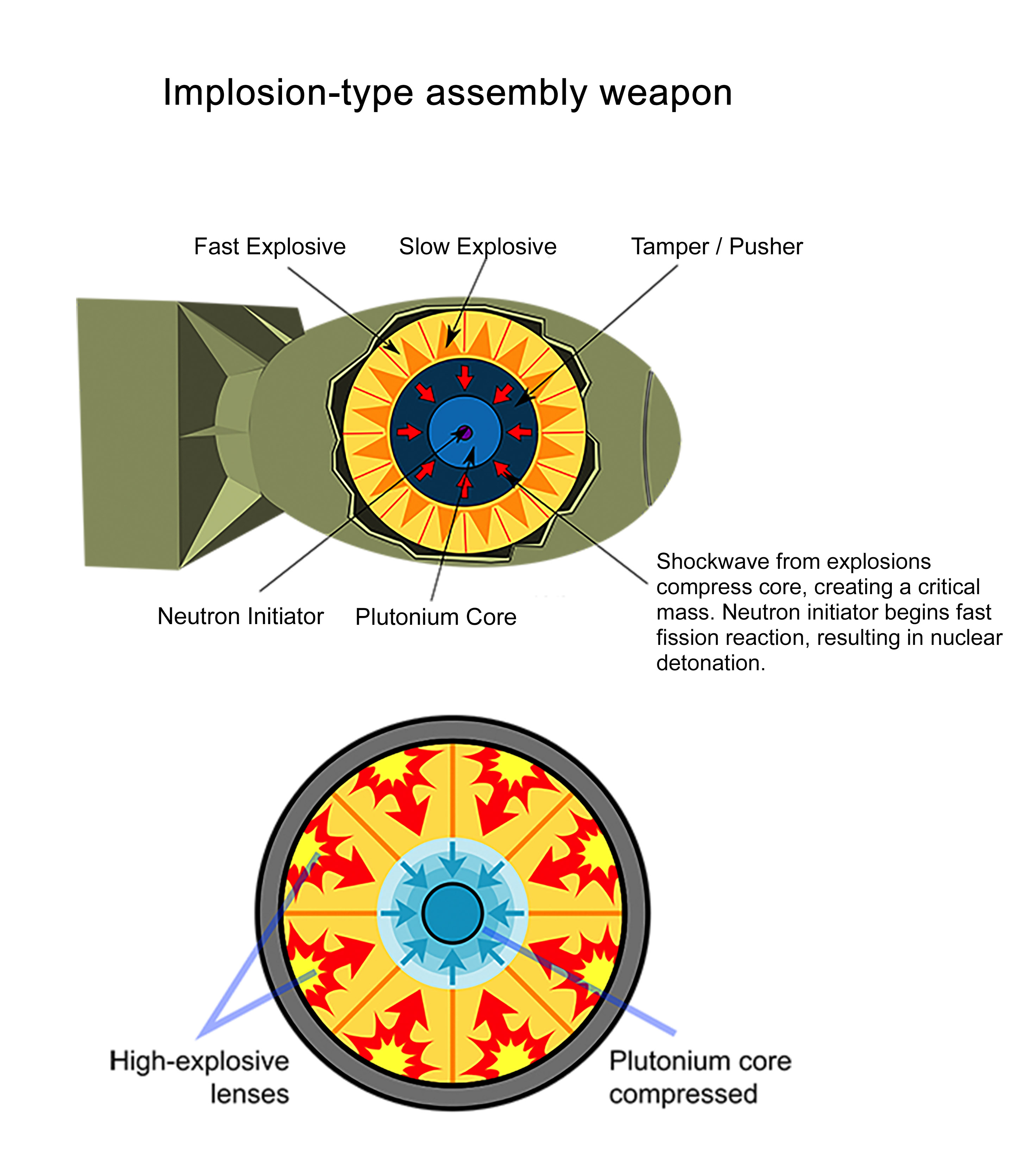
Uranium-235 isn’t very effective in implosion-type devices, as it has a high critical mass requirement – complicating its deployability in military conflict.[44] Plutonium-239, on the other hand, has a much lower critical mass requirement,[45] but only exists in trace amounts on Earth.
Yet the fuel and reprocessing cycles within Light and Heavy Water Reactors provided a convenient method to source plutonium-239 for implosion devices.[46] As these devices can be built small in size (basketball or smaller), this led to future weapons designs that leveraged their immense energy to fuse isotopes of hydrogen together, providing a dramatically more powerful explosion. Thus, the hydrogen bomb was born,[47] as was our reliance on uranium-235 to source the plutonium that makes them possible.
The following image shows the basic stages of the thermonuclear detonation of a hydrogen bomb, employing a Teller-Ulam design:[48]
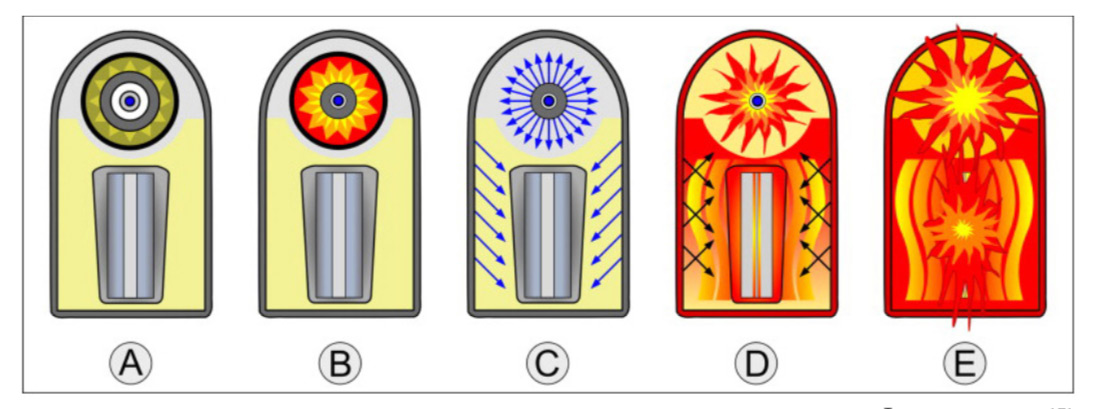
B): The bomb is triggered. The high explosive charges of the primary activate and compress the plutonium core into a super-critical state. A fission detonation occurs.
C). Within the first nanoseconds of detonation, the fission primary emits high levels of X-ray energy that reflect inside of the bomb case. This irradiates the polystyrene foam.
D). The heat and X-ray irradiation cause the polystyrene foam to turn into superheated plasma, which expands massively and compresses the secondary. As this occurs, the excess neutrons from the primary’s fission reaction cause the plutonium in the secondary’s sparkplug to undergo fission, creating even more heat, pressure and radiation.
E). At such extreme heat and pressure, the lithium-6 deuteride separates into tritium and deuterium, which are isotopes of hydrogen. Under these circumstances, these isotopes fuse together to form helium in a reaction thousands of times more powerful than the fission detonations of atomic bombs.
In short: by harnessing the heat, radiation and pressure of an implosion-type fission bomb, thermonuclear weapons fuse isotopes of hydrogen together to create helium, essentially forming a second sun when detonated. With potential yields in the megatons, we can now build weapons that make the bombs dropped on Japan seem like firecrackers in comparison.
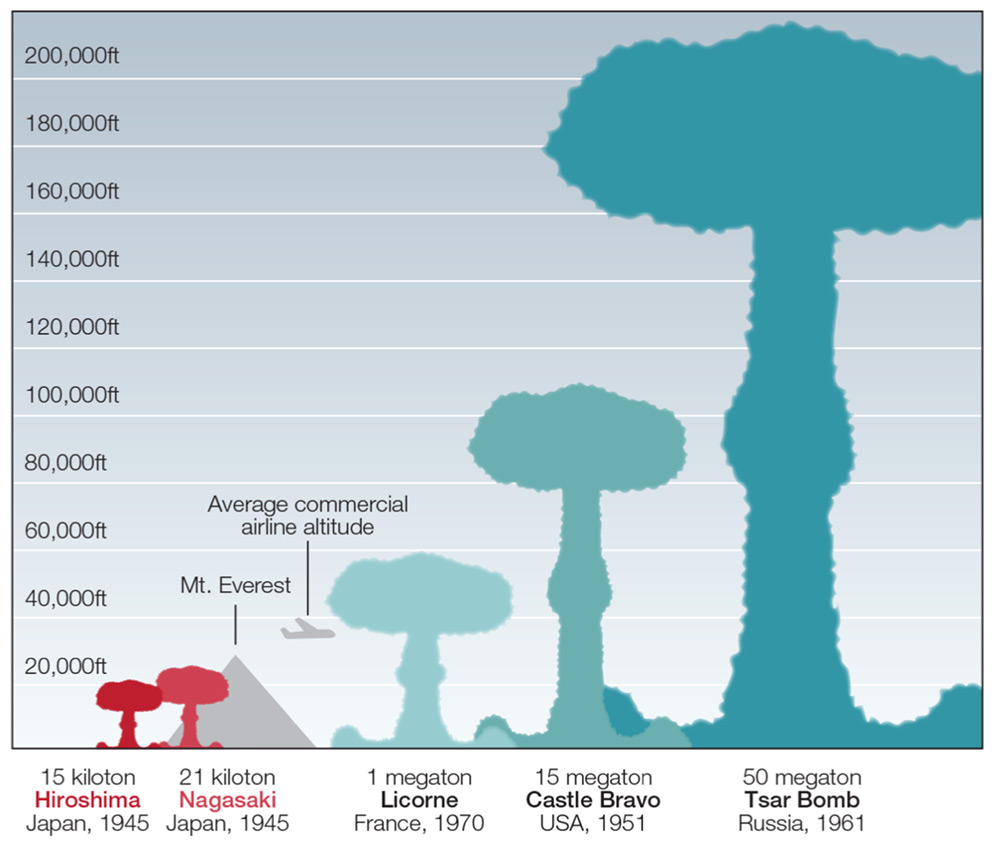
But without plutonium-239 to facilitate a thermonuclear detonation, none of the thousands of hydrogen bombs in the world could exist. And without using Pressurized Water Reactors, there would not be a straightforward way to efficiently produce plutonium-239.
This is why we fuel our power plants today with nuclear dynamite that creates waste products that last for thousands of years and rank among the most toxic substances in existence: to build and maintain nuclear arsenals. That’s the dirty secret behind our approach to nuclear energy. We have corrupted the most powerful energy source that we have ever discovered in the name of an arms race, and at the cost of a world placed in perpetual jeopardy.
If we’re willing to contend with the 15,000 nuclear weapons in global arsenals as enough, and finally forsake the drive to intertwine their production with civilian nuclear energy, thorium gives us another option. One that can avoid nearly every complication with past approaches to atomic power, as well as usher in a clean energy future that exceeds every threshold of our present limitations.