So, What Is A Thorium Reactor?
When people think of nuclear power, they commonly think of a large facility with tall steam stacks, perhaps also containing potentially dangerous materials that could cause calamity under the wrong circumstances. That’s the classic “Pressurized Water Reactor” which works via a combination of pressurized water and enriched uranium. In contrast, Liquid Fluoride Thorium Reactors (LFTRs) use thorium within a high-temperature liquid moderator – no pressure needed – and they work in a way that avoids atomic energy’s most serious problems.[14] Here’s a short list of their highlights:
- LFTRs are highly efficient – hundreds of times more so than Pressurized Water Reactors.[15]
- LFTRs are extremely safe. Because their fuel and reactant are liquid and not under extreme pressure (unlike traditional reactors), it is physically impossible for them to “melt down.”[16]
- Thorium is more stable than other radioactive elements and is safe to handle in raw form unless ingested or inhaled. Additionally, it does not require additional enrichment to power a reactor.[17]
- LFTRs produce far less waste than Pressurized Water Reactors and can also consume both nuclear waste and weapons-grade nuclear material as fuel.[18] Of what small amounts of waste remain, it takes only decades for it to become safe as opposed to millennia with reactors powered by enriched uranium.[19]
- The LFTR’s thorium fuel supply is highly abundant – thorium is about as common as lead – making it thousands of times more plentiful than fuel-grade uranium (only about 0.7% of all uranium in Earth’s known land reserves).[20]
- The thorium fuel in LFTRs is difficult to weaponize. While theoretically possible, the weapon would be unstable, far weaker than traditional nuclear weapons, and would be significantly less practical for use in conflict.[21]
- As a result of their efficiency and safety, LFTRs can be much smaller than Pressurized Water Reactors. Where Pressurized Water Reactors often sit on multi-acre compounds and require large buffer zones in case of emergencies, LFTRs can be around the size of a house or even smaller.[22]
- LFTRs are significantly less expensive to build than Pressurized Water Reactors, and their small size allows them to be mass-produced on assembly lines in a standardized and modular capacity. That means nuclear reactors can become iterations of a product model as opposed to custom-built facilities. The cost savings presented by this capability are immense.[23]
- Although recent thorium designs are experimental, the technology is proven to work both reliably and impressively – an emphasis that will be elaborated upon within a later section of this chapter.
LFTRs are superior to today’s Pressurized Water Reactors in nearly every way possible, and their capabilities have been known to science since the 1960’s.[24] But that prompts an important question: why aren’t we using them today?
To answer that, we’ll need to cover some background that’s easier to understand by first reviewing a few terms surrounding atomic energy. What follows is a quick refresher from science class, or a primer if you’re not familiar with how nuclear power works. (Feel free to skim it, or to skip it now and refer to it as necessary.)
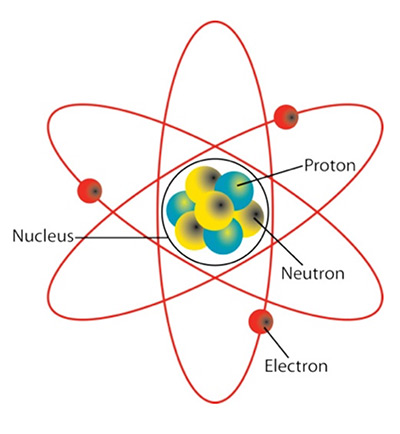 Atom: the building block of matter, composing everything we see and touch. Atoms generally have three types of particles within them. The center of the atom houses the nucleus, which is comprised of a given number of positively charged protons and neutrally charged neutrons. The nucleus is orbited by negatively charged electrons. The different elements in the world are made up of atoms, and each element has a specific atomic arrangement of these particles as shown in the periodic table of elements. Elements and the nature of their atomic composition are the basis of all chemistry and nuclear science.
Atom: the building block of matter, composing everything we see and touch. Atoms generally have three types of particles within them. The center of the atom houses the nucleus, which is comprised of a given number of positively charged protons and neutrally charged neutrons. The nucleus is orbited by negatively charged electrons. The different elements in the world are made up of atoms, and each element has a specific atomic arrangement of these particles as shown in the periodic table of elements. Elements and the nature of their atomic composition are the basis of all chemistry and nuclear science.
The different elements in the world are made up of atoms, and each element has a specific atomic arrangement of these particles as shown in the periodic table of elements. Elements and the nature of their atomic composition are the basis of all chemistry and nuclear science.
Radioactive decay: the process in which an unstable atom spontaneously emits radiation in the form of atomic particles or energy. Any substance that naturally undergoes radioactive decay is considered to be “radioactive.”
Isotope: an unstable variant of an element, usually as a result of radioactive decay and/or something called transmutation (explained next). Isotopes have numerical designations reflective of their atomic composition. For example: uranium-233 and uranium-235 are isotopes of the element uranium.
Transmutation: the process in which one isotope of an element becomes an isotope of another element through nuclear means (like absorbing a neutron).
Fission: the splitting of an atom’s nucleus, releasing tremendous energy and “fission products” (usually radiation + isotopes of other elements). For example: reactors fueled with enriched uranium work by using a neutron to split the nucleus of uranium-235 into kryptonium-92 and barium-141.[25]
Fusion: the joining of atomic nuclei together to form a new element, releasing more energy than even fission. For example: fusing tritium and deuterium (isotopes of hydrogen) into helium, which is how our sun works.[26]
Fissile fuel: an isotope of an element that can undergo fission directly inside a reactor. Uranium-233 and uranium-235 are fissile fuels.
Fertile fuel: an isotope of an element that can’t undergo fission directly, but can if transmuted into a fissile fuel. Thorium is a fertile fuel.
Enrichment: the process of adding greater levels of a radioactive isotope within a nuclear fuel supply. For example: Light Water Reactors use “enriched uranium,” which involves adding more uranium-235 to a fuel supply to sustain fission. Nuclear weapons use “highly enriched” nuclear material to sustain a faster chain reaction. Thorium reactors (especially LFTRs) do not require enrichment.[27]
Breeding: a process in certain reactor designs that employ transmutation to transform a fertile fuel into a fissile fuel. Any reactor that undergoes a breeding process is considered a “breeder reactor.” LFTRs are breeder reactors.[28]
Pressurized Water Reactor (PWR): 1950’s-era reactor designs that use highly pressurized water to help regulate and make possible a fission reaction inside a reactor core. Pressurized Water Reactors use solid fuel and are the most common nuclear reactors operating today.[29]
Light Water Reactor (LWR): a type of Pressurized Water Reactor that uses enriched uranium to generate electricity. Most Pressurized Water Reactors take this form.[30]
Heavy Water Reactor (HWR): a type of Pressurized Water Reactor that does not use enriched uranium, but rather uses a type of water with an extra neutron, known as deuterium oxide or “heavy water,” to sustain fission. These reactors are less common, but still present proliferation risks – especially for weapons-grade plutonium.[31]
Molten Salt Reactor (MSR): a type of advanced reactor design that uses a special type of non-radioactive salt that becomes liquid at high temperatures to act as both a moderator for the reactor and a carrier mechanism for nuclear fuel. They operate at standard atmospheric pressure and have a liquid fuel supply. LFTRs are a highly efficient form of Molten Salt Reactors that also undergo breeding.[32]
With these terms defined, we’ll take a minute to review a bit of our history with atomic energy – specifically addressing why thorium isn’t the primary source of nuclear fuel today.