The Graphene Key
In Chapter Five, we briefly discussed graphene’s potential to make hydrogen storage tanks. But that’s only the start of its capabilities. As a base concept, graphene is a single atom-thick sheet of carbon that takes the shape of a hexagonal lattice. Extraordinarily thin in form, it has three distinct traits:
- Extreme strength. With an overall strength roughly 200 times that of hardened steel, graphene is the strongest-known material on Earth – as well as one of the lightest.[24] It’s also highly resistant to both corrosion and heat, with a melting point in excess of 8,132°F (4,500°C). During a 2008 interview with graphene’s inventor, Professor of Engineering John Home at Columbia University, he remarked:
“Our research establishes graphene as the strongest material ever measured, some 200 times stronger than structural steel. It would take an elephant, balanced on a pencil, to break through graphene the thickness of Saran Wrap.”
- Flexibility. As graphene is formed by sheets of carbon as potentially thin as one atom it can retain a rigid structure or be as flexible as a sheet of paper. It can further function at full strength when assuming a range of shapes, even when designed to bend or stretch.
- Conductivity. Graphene is a lattice of carbon atoms, and in such a form is an excellent conductor of electricity.[25] This enables graphene to lend its strength and flexibility to anything electrical – as a function of either transmission, structure, or storage.
The combination of these three traits make graphene uniquely suited for a wide array of applications that serve critical roles in our economy and society.
They include:
Consumer electronics. As an ultra-strong and ultra-conductive material, graphene can be used to create sophisticated electronics that are highly durable. The following images show prototype mobile phones with graphene screens that are both flexible and thousands of times stronger than today’s phone screens.

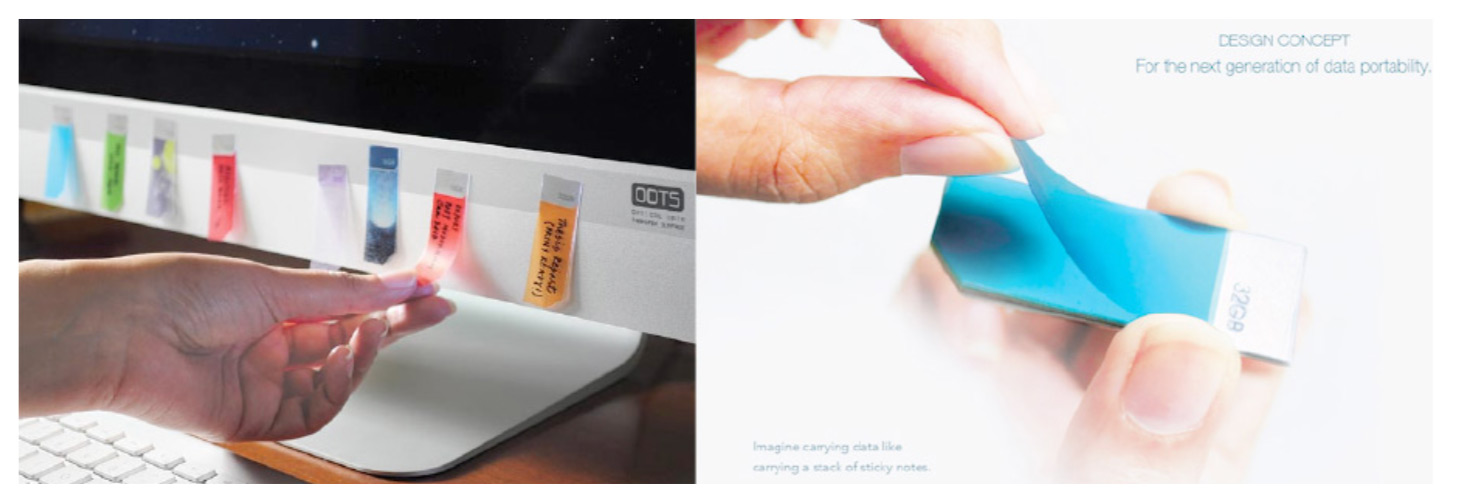
Electronics made with graphene can also store large amounts of data in small physical spaces.[26] The following images show a concept for a new type of jump drive that works like sticky post-it notes,[27] which while not currently in production are well within the realm of technical feasibility.
Graphene’s conductivity also gives it high capacities for data transfer, some 7,000% faster than today’s technology.[28] Researchers have conducted experiments that show graphene antennas can transmit data at speeds of up to 100 terabytes a second.[29] To contextualize, a high-definition feature-length film generally ranges from 3-9 gigabytes in size. There are 1,000 gigabytes in a terabyte. Assuming an average size of 6 gigabytes, this translates to a transfer capacity of approximately 166 high-definition films per-second.
Medicine. High strength and conductivity with low weight and reactivity gives graphene excellent potential for medical applications.[30] Uses include stents to prevent arterial restriction, high strength and lightweight casts for injuries and providing the physical scaffolding to help paralyzed people walk again.
 High-performance energy storage. Graphene is uniquely well-suited to hold energy in capacitors, which work through direct energy storage as opposed to batteries that technically require a chemical reaction to generate a charge.[31] Most portable electricity in our day-to-day lives involve batteries, and they’re generally preferred to capacitors because they normally have a higher “energy density” – the amount of energy stored in a system per unit volume.
High-performance energy storage. Graphene is uniquely well-suited to hold energy in capacitors, which work through direct energy storage as opposed to batteries that technically require a chemical reaction to generate a charge.[31] Most portable electricity in our day-to-day lives involve batteries, and they’re generally preferred to capacitors because they normally have a higher “energy density” – the amount of energy stored in a system per unit volume.
But graphene’s different.
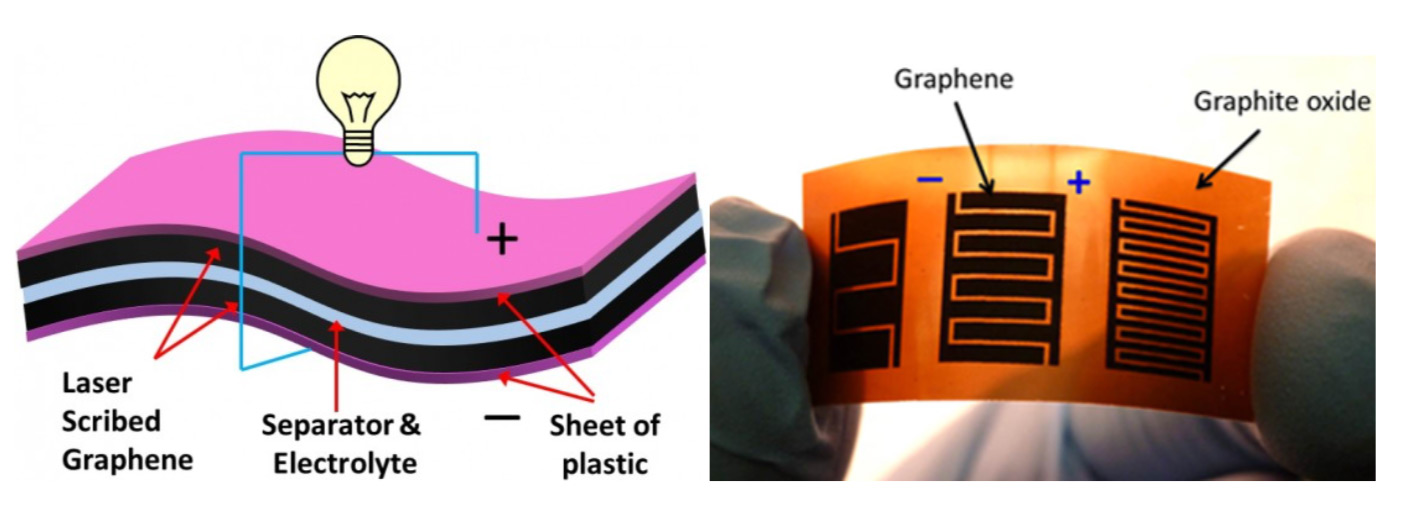
Because it’s so thin and conductive, graphene can form “supercapacitors” that can function at equal to even superior levels than high-performance lithium-ion batteries.[32] These supercapacitors are made by layering sheets of graphene between an electrolyte that’s then encased in non-conductive plastic. The following two images show a theoretical cross-section of a graphene capacitor and a real-world prototype of such a capacitor, respectively.
But these prototypes reflect the relatively new emergence of graphene research. As graphene can be made microscopically thin (theoretically to the scale of an atom), advances in manufacturing can eventually enable us to drastically increase the potential surface area of graphene used for direct energy storage.
To conceptualize: let’s imagine a sheet of printer paper, which is 0.1mm thick.[33] Pretty thin, right? For human eyes, certainly, but not when we’re thinking of a substance that could potentially be microscopically thin. Let’s say that we become able to reliably manufacture insulated graphene capacitance sheets to the thickness of a micron (0.001mm). As a sheet of 8.5x11” printer paper has a thickness of 0.1mm,[34] it would take 100 micron-thick sheets of graphene, then, to comprise one sheet of 8.5x11” printer paper. As there are 25.4 millimeters to the inch, we can derive that a one-inch-thick stack of printer paper would have 254 individual pieces. At 100 graphene sheets per piece of paper, that translates to 25,400 sheets of graphene per inch of thickness.
It’s not uncommon for a standard car battery to have a width and depth similar to a sheet of printer paper. If we were to assume a height of ten inches, we could layer as many as 250,000 graphene sheets in that same volume. At 8.5x11”, a sheet of printer paper has a surface area of about 0.65 square feet. At 250,000 sheets strong, that would amount to a total of 162,500 square feet. A football field, for comparison (including end zones) has a surface area of 57,600 square feet.[35] That means this graphene box would contain the equivalent surface area of 2.5 football fields, yet compacted to the size of a car battery – and made with flexible, non-toxic materials that are 600 times lighter than structural steel and 200 times stronger.
A 2017 paper from The Nanotechnology Journal claimed that graphene supercapacitors today can deliver levels of performance that begin to eclipse industry-grade lithium-ion batteries.[36] Lithium-ion batteries are also much heavier, more expensive and have increased environmental and humanitarian costs.[37] That graphene supercapacitors in their infancy can already compete with high-performance lithium-ion batteries – as well as directly interface with hydrogen fuel cells – further supports their candidacy for future deployment as a solid-state energy solution.
Structural material. Extremely strong. Completely flexible – or reliably rigid. Capable of storing tremendous energy in a tiny footprint. Graphene isn’t revolutionary because it’s impressive in each of these categories, it’s revolutionary because it’s impressive in all of these categories.
Consequently, it can transform the way we build things. Take the electric car for instance.
Instead of an electric car storing packs of heavy batteries, the car’s chassis can be interwoven layers of graphene and can itself be the energy storage medium. When you think of the 162,500 square feet of surface area that could be potentially stored within the size of a car battery, an entire vehicle chassis is a subsequently higher order. We could potentially reach points where we could interweave millions of square feet of energy storage medium within a single electric vehicle – increasing range, reliability and structural integrity at far less weight.

Graphene enables us to wield strength, flexibility in form, and energy storage all at once, each potentially at a higher degree of performance than we can manage with leading commercial solutions today. Tomorrow’s skyscrapers, bridges, public infrastructure, transportation systems – each can also serve as electricity storage. And through Scarcity Zero’s core offerings of electricity, fuel and heat, we have the means to synthesize graphene and other revolutionary materials far easier and at far less expense than we can today.
While this is key to a clean energy future, making things is only one part of the equation. Whatever we construct must in turn be recycled or disposed of in an end-of-life cycle. The framework accounts for this through several approaches on both land – and sea – that can present major environmental improvements.